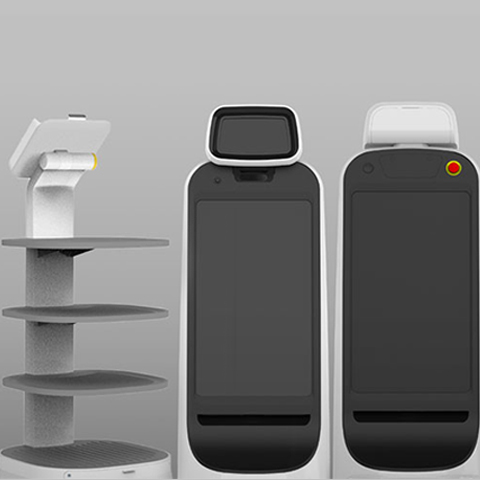
Model Solution Participates in MD&M West 2026, the Largest Medical Device Trade Show in the U.S.
January 29, 2026
■ Showcasing Total Hardware Solutions for Medical Devices at North America’s Leading MedTech Exhibition ■ Presenting Core Component Portfolios for Diagnostics, Cell & Gene Therapy, and Diabetes Care Devices ■ Expanding Strategic Partnerships with Global Bio and Medical Device Companies Model Solution Co., Ltd. (CEO Hyung-min Yoo), a global innovation hardware platform company under the Hankook & Company Group (Chairman Hyun-bum Cho), will participate in Medical Design & Manufacturing West 2026 (MD&M West), the largest medical device trade show in the United States, to be held in Anaheim, California, from February 3 to 5 (local time). MD&M West is North America’s premier exhibition encompassing the full spectrum of medical device design, manufacturing, and engineering. The event brings together leading global MedTech companies, as well as medical device manufacturers and supply-chain stakeholders to exchange insights on the latest technology trends and industry developments. Model Solution has participated in MD&M West on an annual basis, steadily expanding its global medical device customer base—particularly in the United States—while deepening its market expertise. At this year’s exhibition, the company will showcase its comprehensive, end-to-end medical device solution capabilities, covering product planning, development, production, and mass manufacturing. These capabilities are underpinned by Model Solution’s competitive strength in integrating design excellence with precision manufacturing technologies. In addition, the company will showcase a portfolio of core medical device components for diagnostic and analytical medical devices (Diagnostics), medical devices related to Cell & Gene Therapy, and Diabetes Care medical devices, for which the company has participated in development and is currently supplying key components to its customers. “As the global medical device market continues to evolve, demand is increasing for manufacturing partners that can deliver not only advanced design and engineering capabilities, but also stable mass production and robust quality assurance,” said Hyung-min Yoo, CEO of Model Solution. “Leveraging our integrated manufacturing solutions that span from design and prototyping to mass production, we will further strengthen our role as a trusted partner supporting both development and manufacturing for global medical device customers.” Model Solution collaborates closely with global MedTech companies across the entire medical device lifecycle—from early-stage development through mass production—based on its core capabilities in precision machining, injection molding, and cleanroom assembly. The company is also expanding its footprint in the precision medical device components market, including medical robotics, while continuously strengthening its technological expertise and quality competitiveness. Model Solution has obtained ISO 13485, the international quality management standard for medical devices, and has established ISO Class 7 cleanroom production lines, providing an optimized manufacturing environment for high-quality medical devices. In parallel, the company continues to enhance its R&D capabilities and reinforce its competitiveness as a specialized CDMO supporting medical device development and manufacturing. Furthermore, Model Solution has recently expanded strategic collaborations with global bio and medical device companies, including joint development projects with European pharmaceutical companies for next-generation drug delivery devices. Through these partnerships, the company aims to accumulate development and manufacturing experience across major regulated markets and further strengthen its mid- to long-term growth foundation in the medical sector.